FIELD OF RESEARCH / THE RESEARCH GROUP’S NAME
Experimental and theoretical investigation of the antioxidant activity of the biologically active molecules.
SHORT DESCRIPTION OF RESEARCH
The research in our group includes experimental and theoretical investigation of antioxidant and antiradical activity of phenolic, polyphenolic and other molecules (primarily from the group of flavonoids, phenolic acids, and catecholamines). The complementary experimental and theoretical approaches in the investigation of the mechanisms of antioxidant activity of molecules are utilized, including structure-activity relationship (SAR) models with physicochemically meaningful descriptors, in order to determine the possible mechanisms.
SARs models are obtained for the biologically relevant oxygen (hydroxy, peroxy, superoxide anion, ascorbyl and chlorinated methylperoxy), nitrogen (nitrogen monoxide) and model radicals (2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)). Quantitative parameters for the reduction of investigated radicals (EC50 values, antiradical strength, reaction stoichiometry, number of DPPH moles reduced by one mole of the antioxidant molecule, Trolox equivalent (Trolox Equivalent Antioxidant Capacity assays – TEAC) values, (area under EPR maxima) are correlated with the structure of the molecule, mainly the number, type and position of substituents.
Theoretically, the possible antiradical mechanisms are investigated in different solvents. The thermodynamically most probable mechanisms are quantified by the physicochemical descriptors as Bond Dissociation Enthalpy – BDE, Ionization Potential – IP, Proton Dissociation Enthalpy – PDE, Proton Affinity – PA and Electron Transfer Enthalpy – ETE. Along with thermodynamic parameters, the rate constants (calculated by the Transition State theory and Marcus theory approaches) are obtained to describe the most probable mechanism. The results for the antioxidant activity are correlated with the existing data in the literature to develop quantitative structure-activity relationship (QSAR) models which allow the estimation of antiradical activity of molecules with insufficient experimental data or predict the structure of newly synthesized molecules with desired structural characteristics and activity.
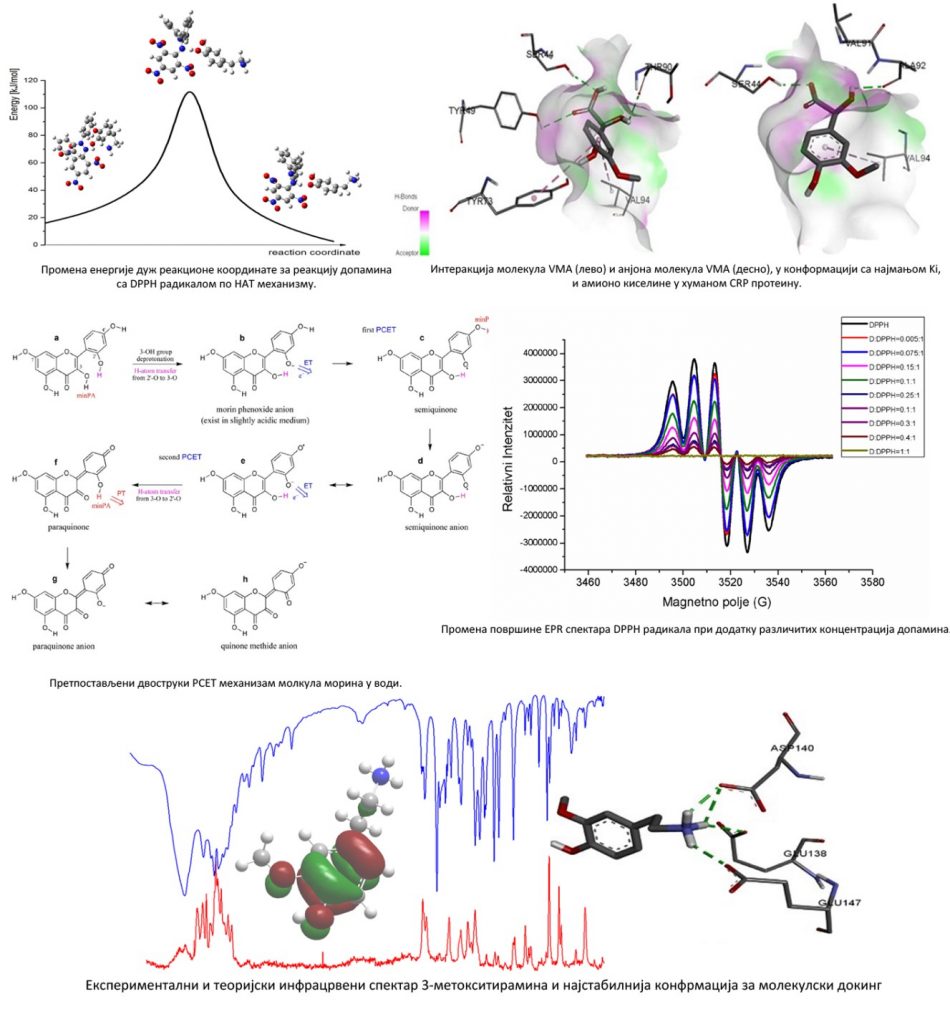
The sensitivity of the fluorescence parameters of biologically active molecules, like proteins, allows the use of fluorescent measurements for the characterization of molecular mechanisms of biological activity of proteins. The conformational and dynamic changes in proteins (bovine and human serum albumin), occurring as a result of binding fluorophores (tested antioxidants) to the protein matrix, are investigated. The influence of the experimental parameters (temperature, pH value …) on the intrinsic fluorescence is also analysed. The distances between the donor (protein) and acceptor (investigated molecule) are obtained from the Förster theory. Based on the thermodynamic state functions, ΔG° (Gibbs free energy), ΔH° (enthalpy) and ΔS° (entropy), the type of interaction between the protein and antioxidant molecule can be determined. The molecular docking, as one of the most widely used theoretical methods in modern, structure-based drug design, is used to determine the conformation of small ligand molecules which are essential for their binding to proteins.
The antibacterial, antifungal and antiquorum activity of the phenolic and polyphenolic molecules allows the investigation of the dependence of cell movement and biofilm formation on the presence of specific antioxidant molecules.
THE MEMBERS OF RESEARCH GROUP
- Jasmina Dimitrić-Marković, Ph.D., full professor
- Dušan Dimić, MSc, teaching assistant
SCIENTIFIC EQUIPMENT
- Raman spectrometer Thermo Scientific DXR Raman microscope
- FTIR spectromete, Avatar 370 -Thermo Nicolet
- EPR spectrometer Bruker Elexsys E540 EPR
- NMR spectrometer Varian Gemini 200 MHz
- UV-VIS spectrometer Thermo Scientific 220
- Cluster MEDFLOW (336 processors and 21 nodes)
COOPERATION AND PROJECTS
Ongoing projects:
- “Dynamics of nonlinear physicochemical and biochemical systems with modelling and prediction of their behaviour in nonequilibrium conditions“ (National project financed by Ministry of Education, Science and Technological Development of the Republic of Serbia, project number 172015)
- “Structure and dynamics of molecular systems in ground and excited electronic states“, (National project financed by Ministry of Education, Science and Technological Development of the Republic of Serbia, project number 172015)
Cooperation:
- State University of Novi Pazar, Republic of Serbia
- Faculty of Science – University of Kragujevac, Republic of Serbia
- Bioengineering Research and Development Center – BIOIRC, Kragujevac, Republic of Serbia
- Karolinska Institute, Stockholm, Sweden
- Institute “Ruđer Bošković”, Zagreb, Republic of Croatia
- Faculty of Agriculture – University of Osjek, Republic of Croatia
- Torlak, Institute for immunology and virusology, Belgrade, Republic of Serbia
SELECTED PUBLICATIONS
- Ana Amić, Bono Lučić, Višnja Stepanić, Zoran Marković, Svetlana Marković, Jasmina M. Dimitrić Marković, Dragan Amić, Free radical scavenging potency of quercetin catecholic colonic metabolites: thermodynamics of 2H+/2e- processes, Food Chem. 218, 144-151, 2017.
- Jasmina M. Dimitrić Marković, Boris Pejin, Dejan Milenković, Dragan Amić, Nebojša Begović, Miloš Mojović, Zoran S. Marković, Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: the energy requirements calculations as a prediction of the possible antiradical mechanisms, Food Chem. 218, 440-446, 2017.
- Jelena Tošović, Svetlana Marković, Jasmina M. Dimitrić Marković, Miloš Mojović, Dejan Milenković, Antioxidative mechanisms in chlorogenic acid, Food Chem. 237, 390-398, 2017.
- Dušan Dimić, Dejan Milenković, Zoran Marković and Jasmina Dimitrić Marković, The Antiradical Activity of Catecholamines and Metabolites of Dopamine: Theoretical and Experimental Study, PCCP, 19, 12970-12980, 2017.
- Dušan Dimić, Dejan Milenković, Zoran Marković, Jasmina Dimitrić Marković, Structural and Spectral analysis of 3-Methoxytyramine, an Important Metabolite of Dopamine, Journal of Molecular Structure, 1134, 226-236, 2017.
- Ana Amić, Zoran Marković, Jasmina M. Dimitrić Marković, Svetlana Jeremić, Bono Lučić, Dragan Amić, Free radical scavenging and COX-2 inhibition by simple colon metabolites of polyphenols: A theoretical approach, Computational Biology and Chemistry 65, 45-53, 2016.
- Ana Amić, Zoran Marković, Jasmina M. Dimitrić Marković, Bono Lučić, Višnja Stepanić,Dragan Amić, The 2H+/2e_ free radical scavenging mechanisms of uric acid: thermodynamics of NAH bond cleavage, Computational and Theoretical Chemistry, 1077, 2-10, 2016.
- Boris Pejin, Ana Ćirić, Jasmina Dimitrić Marković, Jasmina Glamočlija, Miloš Nikolić, Marina Soković: An insight into anti-biofilm and anti-quorum sensing activities of the selected anthocyanidins: the case study of Pseudomonas aeruginosa PAO1, Natural Product Research, DOI:10.1080/14786419.2016.1222386.
- Boris Pejin, Ana Ciric, Jasmina Dimitric Markovic, Jasmina Glamoclija, Milos Nikolic, Bojana Stanimirovic and Marina Sokovic, Quercetin Potently Reduces Biofilm Formation of the Strain Pseudomonas aeruginosa PAO1 in vitro, Current Pharmaceutical Biotechnology, 733-737, 2015.
- Miloš Filipović, Zoran Marković, Jelena Đorović, Jasmina Dimitrić Marković, Bono Lučić, Dragan Amić, QSAR of the free radical scavenging potency of selected hydroxybenzoic acids and simple phenolics, Comptes rendus chimie 18, 492-498, 2015.
- Dušan Dimić, Andrew Mercader, Eduardo Castro, Chalcone derivatives cytotoxicity activity against MCF-7 human breast cancer cells QSAR study, Chemometrics and Intelligent Laboratory Systems, 2015, 146, pp: 378-384, DOI: 10.1016/j.chemlab.2015.06.011
- Ana Amić, Zoran Marković, Jasmina M. Dimitrić Marković, Višnja Stepanić, BonoLučić, Dragan Amić, Toward an improved prediction of the free radical scavenging potency of flavonoids: The significance of double PCET mechanisms, Food Chem. 152, 578-585, 2014.
- Jasmina M. Dimitrić Marković, Dejan Milenković, Dragan Amić , Miloš Mojović, Igor Pašti Zoran S. Marković, The preffered radical scavenging mechanisms of fisetin and baicalein towards oxygen-centred radicals in polar, protic and aprotic, solvents, RSC Advances, 4, 32228-32236, 2014.
- Zoran Marković, Jelena Đorović, Jasmina M. Dimitrić Marković, Dragan Amić, Investigation of the radical scavenging potency of hydroxybenzoic acids and their carboxylate anions, Monatshefte fur Chemie Chemical Monthly, 145, 953-962, 2014.
- Zoran Marković, Dragan Amić, Dejan Milenković, Jasmina M. Dimitrić Marković, Svetlana Marković, Examination of the chemical behavior of the quercetin radical cation in basic media, PCCP, 15, 7370-7379, 2013.
- Jasmina M. Dimitrić Marković, Zoran S. Marković, Jugoslav. B. Krstić, Dejan Milenković, Bono Lučić, Dragan Amić, Interpretation of the IR and Raman spectra of morin by density functional theory and comparative analysis, Vibrational Spectroscopy, 64, 1-9, 2013.
- Dragan Amić, Višnja Stepanić, Bono Lučić, Zoran Marković, Jasmina M. Dimitrić Marković, PM6 study of free radical scavenging mechanisms of flavonoids: Why is the O–H bond dissociation enthalpy able to effectively represent free radical scavenging activity? J. Mol. Model. 19, 2593-2603, 2013.
- Zoran Marković, Dejan Milenković, Jelena Đorović, Jasmina M. Dimitrić Marković, Višnja Stepanić, Bono Lučić, Dragan Amić, PM6 and DFT study of fee radical scavenging activity of morin, Food Chem. 134, 1754–1760, 2012.
- Jasmina M. Dimitrić Marković, Zoran S. Marković, Igor Pašti, Tanja P. Brdarić, Ana Popović Bijelić, Miloš Mojović, A joint application of spectroscopic, electrochemical and theoretical approaches in evaluation of the radical scavenging activity of 3-OH flavones and their iron complexes towards different radical species, Dalton Trans. 41, 7295-7303, 2012.
- Zoran Marković, Dejan Milenković, Jelena Đorović, Jasmina M. Dimitrić Marković, Višnja Stepanić, Bono Lučić, Dragan Amić, Free radical scavenging activity of morin 2’-O− phenoxide anion, Food Chem. 135, 2070-2077, 2012.
- Zoran S. Marković, Svetlana Marković, Jasmina M. Dimitrić-Marković, Dejan Milenković, Structure and Reactivity of Baicalein Radical Cation, Int. J. Quantum Chem. 112(8), 2009-2017, 2012.
- Leposava A. Pavun, Jasmina M. Dimitrić Marković , Predrag T. Đurđević, Milena D. Jelikić-Stankov, Daniela B. Đikanović, Andrija R. Ćirić, Dušan L. Malešev, Development and validation of a fluorometric method for the determine action of hesperidin in human plasma and pharmaceutical form, J. Serb. Chem. Soc. 77 (11) 1625–1640, 2012.
- Jasmina M. Dimitrić Marković, Zoran S. Marković, Tanja P. Brdarić, Vesna M. Pavelkić, Milka B. Jadranin, Iron Complexes of Dietary Flavonoids: Combined Sectroscopic and Mechanistic Study of the Free Radical Scavenging Activity, Food Chem. 129, 1567-1577, 2011.
- Jasmina M. Dimitrić Marković, Zoran S. Marković, Tanja P. Brdarić, Nenad D. Filipović, Comparative spectroscopic and mechanistic study of chelation properties of fisetin and iron in aqeous buffered solutions. Implications on in in vitro antioxidant activity, Dalton Trans. 40, 4560–4571, 2011.
